
|
(S-4) The Many Colors of Sunlight
Perceived Color Even with the rainbow explained, the puzzle of color still baffled scientists. The riddle was solved around 1860 by James Clerk Maxwell (pictured on the left), the brilliant Scottish physicist who also gave us the basic equations of electricity, the ones that predicted electromagnetic waves (see Section S-5). Maxwell showed, while still a student, that two kinds of color existed, depending on whether it was perceived by an instrument or by the human eye:
Even with the rainbow explained, the puzzle of color still baffled scientists. The riddle was solved around 1860 by James Clerk Maxwell (pictured on the left), the brilliant Scottish physicist who also gave us the basic equations of electricity, the ones that predicted electromagnetic waves (see Section S-5). Maxwell showed, while still a student, that two kinds of color existed, depending on whether it was perceived by an instrument or by the human eye:
An instrument using prisms ("spectrograph") will reveal that the human eye can be fooled: different combinations of rainbow colors may look the same to the eye. Our eye contains three kinds of light-sensitive cells, each sensing a different band of colors--one band centered in the red, one in the green and one in the blue. Any color which we see--including brown, olive-green and others absent in the rainbow--is an impression our brain conveys as it combines signals from these 3 color bands. Color-blind persons lack some types of eye cells, and their world lacks certain colors, or even (for those having only one kind of cell) any color at all. Color blindness is much more prevalent in men; in women, on the other hand, a rare mutation exists whose eyes have four different kinds of receptor cells. The rest of us can only guess what colors those ladies must be able to see! [Birds, too, seem to have 4-color vision: see What Birds See by Timothy H. Goldsmith, Scientific American, July 2006, p. 69-75. Humans can see red but deer can't, so deer hunters wear red jackets for their own safety, without their prey noting.]That is why color TV and color printers can be based on the three "primary colors" red, green and blue, or 3 combinations of those colors. Such 3-color combination will not in any way reproduce the true spectral color of the objects they show, but for most colors, our eyes alone won't notice a difference. However, given any choice of 3 "basic" colors, we usually cannot mimic any color perceived by the eye. An e-mail discussion of this (involving physics teachers) is linked here.
You can find on the web programs which let you experiment with 3-color combinations, using the color monitor of your computer. A somewhat simple program for doing so was provided with the original version of this web page, but be aware that it requires you to go into the "source code" of the HTML page, the text page which specifies the contents of the web page. (You will get instructions). It is linked at the end of this web page.
(1) In light emitted from solids, liquids or extensive bodies of dense gas such as the Sun, the colors are distributed continuously. Their exact distribution ("black body spectrum") depends on the temperature at which it is produced--a warm hand radiates mostly in the infra-red, a glowing bar of iron is cherry-red, a lightbulb filament is bright yellow, and sunlight is white-hot.
|
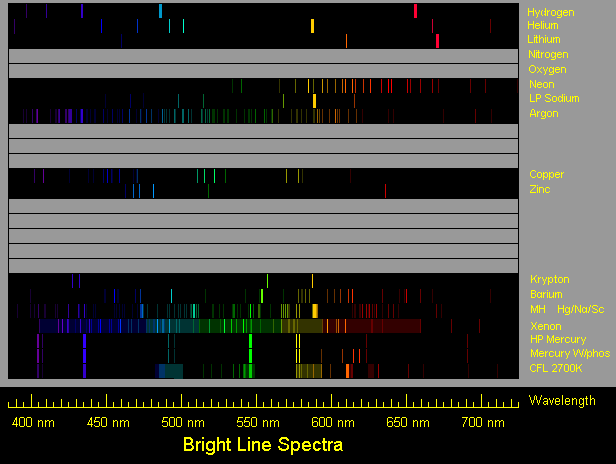 |
| Spectra of selected elements, © Donald E. Klipstein (see here for more) |
-
After this web page was written, I added one for trying out three-color combinations
(S4-A) Experimenting with Colors .
Be aware, however, that it involves going into the source code of that page and modifying it. If you are learning HTML, you might find it useful. Otherwise, the web page linked earlier is much easier to use.
Next Stop (starting on the Doppler effect)
(S-4A-1) The Velocity of Light
or else
(S-5) Waves and Photons
Timeline Glossary Back to the Master List
Author and Curator: Dr. David P. Stern
Mail to Dr.Stern: stargaze("at" symbol)phy6.org .
Last updated: 9-23-2004
R-formatted 26 March 2006

