Lesson Plan #39 http://www.phy6.org/Stargaze/Lsun4spe.htm
(S-4) The Many Colors of Sunlight |
Part of a high school course on astronomy, Newtonian mechanics and spaceflight
by David P. Stern
|
This lesson plan supplements: "The Many Colors of Sunlight," section #S-4: on disk Sun4spec.htm, on the web
http://www.phy6.org/stargaze/Sun4spec.htm
"From Stargazers to Starships" home page and index: on disk Sintro.htm, on the web
|
|
Goals: The student will learn
Note: This lesson can also be embedded in a course on optics. At this level, the student is told that the wave nature and wavelength are deduced from the way parts of a beam of light resonate with each other. The question of why these waves are called "electromagnetic" is left for the next lesson.) Terms: [Refraction of light, dispersion of light], spectrum, spectral color, perceived color, 3-color theory, black body spectrum, spectral lines, diffraction grating, wave, wavelength, absorption spectrum. Stories: Discovery of helium. Starting the lesson:
One striking phenomenon involving color is the rainbow. People generally divide the rainbow into 7 colors. Anyone knows what they are, in the order in which they appear? (Students may answer--put on board, make sure class copies). Red, orange, yellow, green, blue, indigo, violet (All of you know "indigo"? It is the name of a dye. What color are blue jeans?
We obtain the same sequence when light passes a prism--red is bent least, violet the most (draw on board). Anyone knows why? (students may answer)
When a beam of light hits a glass windowpane (draw on the board) with two parallel sides, the beam is refracted one way as it enters the glass, then the opposite way as it leaves. The net result is that it emerges with the same direction as before. So, inside the glass diffect colors may be separated, but when they emerge again they all move again in the same direction, and no separation remains.
(optional) How about rainbows? When do you see rainbows?
Has anyone noticed where in the sky the rainbow appears?
Sometimes you see a second, outer rainbow--fainter, larger, with red now the innermost color. Any guess how it gets produced? Let me give you a hint. When a beam of light goes through a windowpane (refer the drawing on the board), not all the light goes through. Some of it, instead of being refracted the second time, is reflected back, as if the back of the glass was a mirror. (add the reflected beam to the drawing on the board, a broken line.)
Given this hint--does anyone know how the colors arise, or can you guess?
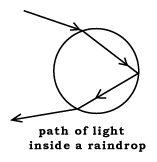
(Teacher) How did Newton show that white light was a mixture of all colors?
(Teacher) In 1800 William Herschel, the astronomer who discovered the planet Uranus, made the following experiment. He split a beam of sunlight using a prism, and let the spectrum coming out of it--the spread of colors--fall on a table. (Draw on the board, adding details as described below.) He then placed a thermometer on the place where the spectrum fell, and noted that the temperature rose. He then moved the thermometer past the red edge of the spectrum, and noted that even there a higher temperature was recorded. What did this mean?
(Teacher) So we have now 7 colors in the rainbow, plus perhaps others which we cannot see (but instruments can). Now let us look at a TV screen. In a TV with a black-and-white picture tube, the screen contains many small dots which glow when a beam of electrons passes them. The beam sweeps over the picture many times each second, and by controlling its strength, each dot it passes over can be made to shine brightly, dimly or not at all. This way a picture is painted on the screen. The newer flat screens have no electron beams, but the glowing dots are directly connected electrically--otherwise it is very similar.
Anyone knows how a color TV gets its color?
But then we can only see three possible colors! How can we see all colors of the rainbow?
Does combining yellow and red produce orange?
Who discovered the "three color theory"--that the eye sees everything as the combination of 3 colors?
What did he show happened when all 3 colors were put together (in proper proportion?)
What is a spectrum?
From now on we only discuss "spectral color" as seen by a prism instrument ("spectrometer"), rather than color observed by the eye.
You are given a closed box with a hole, from which a beam of light emerges. You are given a spectrometer and asked to tell whether the light comes from a filament lightbulb or a fluorescent lamp. How can you tell?
(The fluorescent coating inside the tube absorbs light and re-emits it in a broad continuous spectrum, so in addition to spectral lines, you will also see a continuous spread.)
The star Antares (in the constellation of Scorpio) is reddish, Sirius is white and our Sun is yellow by comparison. How would the surface temperature of the Sun compare to the ones of the two stars?
When the battery in your flashlight runs down, the light it produces looks more orange than yellow. Why?
Why are most flames yellow?
(optional, by the teacher) The polar aurora or "northern lights" is emitted by the high atmosphere (around 100 km or 60 miles) when beams of fast electrons from space hit the edge of the atmosphere. These electrons are guided by the Earth's magnetic field lines and therefore are generally observed only in an "auroral zone" around the magnetic pole, typically 2500 km from the pole. At locations like Fairbanks, Alaska, aurora is not all that rare. (See here for more about the aurora, and here about the auroral zone.) Light is emitted when those electrons collide with atoms, a bit like the way light is emitted when a beam of fast electrons inside a TV picture tube hits the screen. Different spectral lines are observed, but the brightest and most common one is a green spectral line at a wavelength of 5.577 micron. A red line at 6.300 micron is also sometimes emitted. These emissions used to puzzle scientists, because they failed to match any spectra in the laboratory. Did the upper atmosphere contain any new unknown substance?
Around 1925, the answer was found--both colors came from oxygen. When an oxygen atom was activated ("excited") by a collision in a certain way, it took about half a second before it emitted light, an unusually long time. In laboratory experiments, the gas used was so dense that other atoms usually collided with the excited atom and carried away its extra energy. Only high in the atmosphere could the emission process proceed undisturbed. lines.)
A spectrometer can use a prism to separate light into its other components. Do any other ways exist?
|
 (Optional)
If time allows, students can be shown how a diffraction grating works.
(Optional)
If time allows, students can be shown how a diffraction grating works. The grating acts like many closely spaced slits: the light hitting between the slits is scattered irregularly, and only what hits the slits goes through. If light acts as a wave, one can show mathematically that each slit acts as a new source of the wave.
Suppose light arrives at the grating from a direction perpendicular to it. Waves have peaks and valleys, and when the arriving wave-front is at a peak, all slits also start their "local waves" with a peak. Suppose we continue in the same direction 1, 2, 3... wavelengths. The wave from each slit will then also have a peak at those locations. These peaks would be in the same distances if the wave passed intact through the grating, as if it wasn't there, suggesting that much of the wave goes straight through, with no modification. But wait! If each slit is the source of a wave spreading in all directions, what about the part spreading in slanted directions?
Except... if the angle θ is such that the distances of the wave-front from two neighboring slits differ by exactly one wavelength λ . In that case, the distances from the next slits in line are 2λ, 3λ ... and so forth, and the waves continue propagating "in step." That is "constructive interference" between the waves, and in those directions, you will see a fairly bright beam of light.
|
|
As the second drawing shows, if D is the spacing between two neighboring slits, this requires
Note that the angle θ at which the light undergoes constructive interference depends on the wavelength λ, that is, on the spectral color of the light. Each color is therefore bent by a different angle--just as it is in a prism. Because most of the beam goes straight through, the light may not be as bright as in a prism, but the separation of neighboring colors may be much more sensitive.
The above formula allows the light's wavelength to be calculated. For example: you observe the yellow line of sodium with a grating having 1000 lines per centimeter, and find that light is brightest at an angle θ =36°. What is the wavelength λ? In fact, methods based on interference have measured wavelengths with such accuracy, that the international meter--originally defined by two scratches on a bar, kept in a vault in Paris--was at one time redefined in terms of the wavelength of a certain emission. Laser disks for recording songs, videos or data for computers, shimmer in colors, because they contain many closely spaced grooves, which make them act like a grating. The light is reflected from the grooves (with the help of an aluminum backing), rather than passing through, but the effect is very similar. Another interference effect are the colors seen when a thin layer of kerosene floats on a puddle of water--layers with a thickness of the order of a wavelength of light. Some light is reflected from the top of the kerosene layer, some from its bottom (which is the top of the water), and the two reflected waves interfere with each other. For some colors the interference is destructive, for others, constructive, leading to a shimmering of colors. (end of optional section)
What does the spectrum of sunlight tell about the Sun?
|
Back to the Lesson Plan Index Back to the Master Index
Guides to teachers... A newer one An older one Timeline Glossary
Author and Curator: Dr. David P. Stern
Mail to Dr.Stern: audavstern("at" symbol)erols.com .
Last updated: 20 November 2004
 Because (draw on board, students copy) light hitting a flat surface of glass gets bent by an angle. (That is known as refraction--"The beam of light is refracted.")
Because (draw on board, students copy) light hitting a flat surface of glass gets bent by an angle. (That is known as refraction--"The beam of light is refracted.") In a prism (refer to drawing already on the board), the entry side and the exit side are inclined, not parallel, and therefore the direction is changed--by a different amount for each color.
In a prism (refer to drawing already on the board), the entry side and the exit side are inclined, not parallel, and therefore the direction is changed--by a different amount for each color. 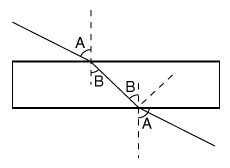 Now, the big question: why the colors?
Now, the big question: why the colors? 
